Nasisoft

Nasisoft ir medicniskā ierīce intranazālai lietošanai un lietošanai inhalācijās.
Nasisoft attīra un mitrina deguna gļotādu lietojot profilaktiski veseliem cilvēkiem, kā arī pacientiem ar iekaisīgām elpceļu slimībām.
Nasisoft atvieglo elpošanu, samazinot deguna un deguna blakusdobumu gļotādas tūsku un kairinājumu, kā arī veicina ātrāku krēpu izdalīšanos.
Sastāvs. 1ml šķīduma satur:
- Nātrija hialuronātu 0,1 mg;
- Nātrija hlorīdu 9,0 mg.
Iepakojums:
- 4ml šķīduma iepriekš pildītā polimēra konteinerā.
- Katrā iepakojumā N10 polimēra konteineri ar sterilu šķīdumu.
INSTRUCTION FOR USE FOR NASISOFT Sterile solution for inhalation and intranasal administration
Description
NASISOFT is а sterile solution for inhalation by nebulizer and/or intranasal administration by direct instillation from the container into the nasal cavities. It is а natural isotonic saline solution in а concentration adjusted to be compatible with human body liquids. NASISOFT is used for mo1stening the respiratory tract. including cases when such cold symptoms are manifested as а cough and nasal congestion.
Qualitative composition
- Sodium chloride – 9.0 mg/ml
- Sodium Hyaluronate – 1.0 mg/ml
- Water for injection
Classification of the product
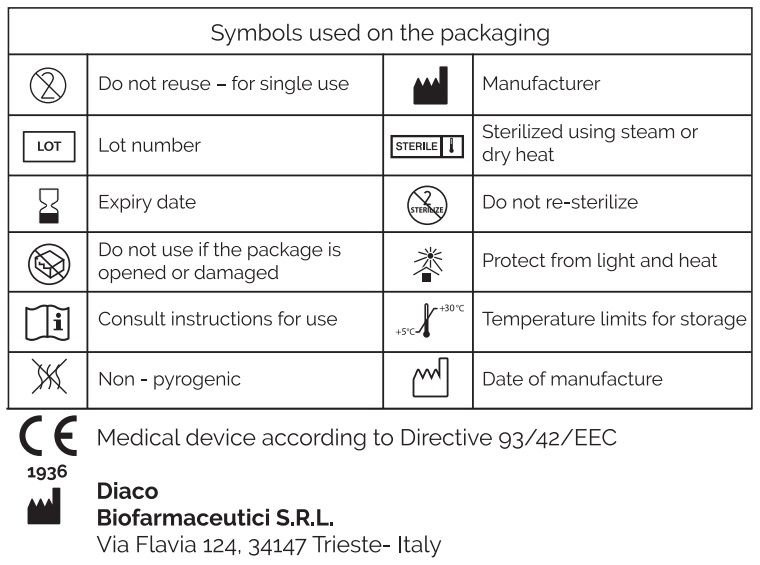
Medical device – sterilized and pyrogen-free – Class ІІа.
Package
4 ml in pre-filled disposable polymer containers, each package containing 0.9% saline solution. Each package contains:
- 10 plastic containers with sterile NASISOFT solution;
- Or 60 plastic containers with sterile NASISOFT solution
Only the contents of the package are sterile. The medical device has been sterilized bу steam sterilization.
lndications
NASISOFT, when used according to the instructions, may be administered to newborns, children, pregnant and nursing women, and adults, for the following intended uses:
- For nasal cavity care and for use in patients with diseases of nasal mucosa, accompanied bу dryness of the nasal mucosa or by mucus formation (atrophic, allergic, infectious, drug-induced rhinitis).
- То eliminate dryness of the nasal mucous membrane caused by being in air-conditioned premises, including air-conditioned cars or centrally-heated premises and on flights.
- After nasal cavity surgery.
Route of administration
By inhalation
The solution can be inhaled by means of а nebulizer using а facemask, an actuator or а nasal cannula, and following the nebulizer manufacturer’s instructions for use.
Use one, 4 ml vial twice а day or according to medical prescription.
By intranasal administration
Intranasal administration of the solution can be performed by squeezing the plastic container and allowing the content to flow into each nasal cavity. Use one. 4 ml vial from 1 to 4 times а day or according to medical prescription.
Apply for up to 4 weeks or according to medical prescription.
Warnings and special precautions for use
- The solution is only intended for use via inhalation or intranasal administration. Do not inject.
- Do not use if the solution is not transparent and colorless.
- Do not use after the expiry date.
- Do not use if the package is opened or damaged.
- The product must be used immediately after opening.
- The product is intended for single use. After use. the unused product is no longer sterile.
- Do not reuse. Once the product has been used for the first time, any residuals of the product are not suitable for repeated use as the product is no longer sterile.
- Do not re-sterilize. Repeated sterilization may cause cross contamination.
- Do not freeze.
- The product must not be administered orally.
- Use by children should be under the supervision of adults.
- This product includes small parts. which can pose а choking hazard. Always keep this product out of the reach of children under the age of three.
Side effects
NASISOFT is contraindicated in case of individual hypersensitivity to any component of the product. In persons with individual intolerance to the solution components allergic reactions are possible.
Isotonic saline solution can cause reversible constriction of the bronchia when inhaled by very sensitive patients with bronchial asthma or а hyperreactive bronchial system. In particularly susceptible subjects, pre-medication with bronchodilators that can help prevent bronchospasmic reactions is advisable at the start of the treatment. Premedication with bronchodilators must be carried out under the control of а doctor.
In case of the onset of bronchospasms or persistent cough, interrupt the treatment and inform the treating doctor.
How to use
For inhalation by nebulizer:
- Check the expiry date indicated on the package. Do not use after the expiry date.
- Take one of the plastic containers with NASISOFТ from the strip of 10 plastic containers.
- Open the plastic container by turning the stopper.
- Use the solution in conjunction with the nebulizer following the device manufacturer’s instructions for use.
For intranasal administration:
- Check the expiry date indicated on the package. Do not use after the expiry date.
- Take one of the plastic containers with NASISOFТ from the strip of 10 plastic containers.
- Open the plastic container by turning the stopper.
- Squeeze the solution from the plastic container inside the nasal cavities.
Shelf life
2 years in intact packaging.
How to store
Store at а temperature from 5℃ to 30℃ (inclusive), away from direct light and heat sources, in adequately closed packaging. The expiry date applies to the product when correctly stored in intact packaging.
Disposal
The product must be disposed of in accordance with applicable laws on municipal waste.