Nasisoft

The Nasisoft is a medical device for intranasal and inhalation use. It exhibits cleansing and moistening properties.
The Nasisoft cleans and moistens nasal mucosa both in healthy persons, and in patients with inflammatory respiratory diseases.
The Nasisoft lightens the breathing due to thinning the secret produced by nasal and paranasal sinus mucosa, and contributes to faster expectoration.
Formula. 1 ml of solution contains:
- Sodium hyaluronate 0,1 mg;
- Sodium chloride 9,0 mg.
How supplied:
- 4 ml in pre-filled disposable polymer containers.
- Each package contains 10 plastic containers with sterile solution.
WHERE TO BUY?
NASISOFT Medical Device Instructions for Use
NASISOFT
Sterile solution for inhalation and intranasal administration
Description:
NASISOFT is a sterile solution for inhalation using a nebulizer and/or for intranasal administration by direct instillation from a polymer dispenser into the nasal passages. It is a natural isotonic saline solution with a concentration compatible with human body fluids.
Purpose of use:
The NASISOFT solution is used for daily moisturizing and care of the respiratory tract, as well as during colds with symptoms such as nasal congestion and cough.
Composition:
- Sodium chloride – 9.0 mg/ml
- Sodium hyaluronate – 1.0 mg/ml
- Sterile water
Product classification:
Sterilized and apyrogenic Class IIb medical device.
Primary packaging:
4 ml pre-filled single-use polymer dispenser, each containing 0.9% saline solution and 0.1% sodium hyaluronate.
Secondary packaging:
- 10 polymer dispensers (4 ml each) with sterile NASISOFT solution, or
- 60 polymer dispensers (4 ml each) with sterile NASISOFT solution.
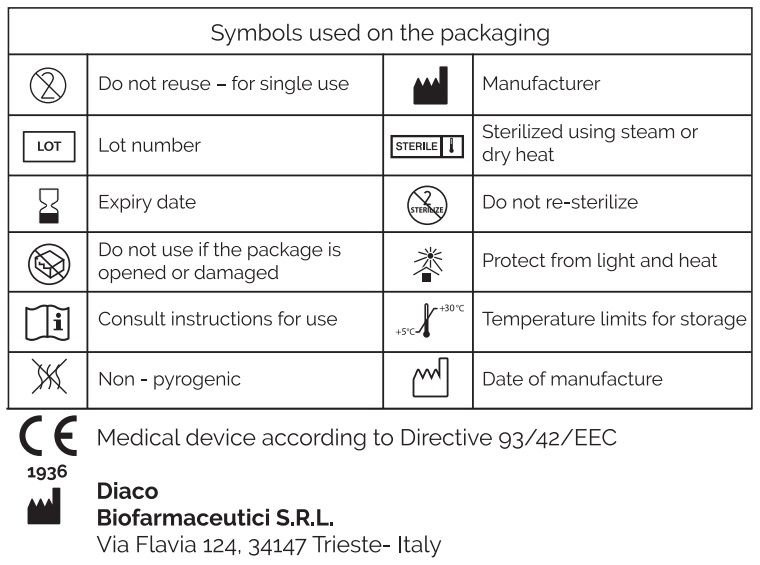
Only the contents of the package are sterile.
The medical device is sterilized by steam sterilization.
Indications:
According to the instructions, the NASISOFT solution is suitable for use in newborns, children, pregnant and breastfeeding women, as well as adults, in cases where the use of the solution is indicated:
- For daily nasal cavity care and for patients with nasal mucosa diseases characterized by dryness or mucus formation (atrophic, allergic, infectious, or drug-induced rhinitis).
- For moisturizing the nasal mucosa when air conditioning or heating is used indoors, in air-conditioned cars, and in other cases that promote drying of the respiratory mucosa, such as during flights.
- After nasal cavity surgeries.
Directions for use:
Inhalation:
Use with a nebulizer according to the device manufacturer’s instructions.
Recommended dose: one 4 ml polymer container twice daily or as directed by a physician.
Intranasal administration:
The solution can be instilled by squeezing the opened polymer container and allowing the contents to flow into each nostril.
Use one 4 ml polymer container per application, one to four times daily, or as directed by a physician.
Duration of use:
Up to 4 weeks, or as directed by a physician.
Warnings and precautions:
- The solution is intended for inhalation or intranasal administration only. Do not inject.
- Do not use if the solution is not clear and colorless.
- Do not use after the expiry date.
- Do not use if the polymer dispenser is open or damaged.
- An opened polymer dispenser must be used immediately after opening.
- Each polymer dispenser is for single use only; any remaining solution is no longer sterile.
- Do not re-sterilize. Re-sterilization may cause cross-contamination.
- Do not freeze the solution.
- The solution is not intended for oral use.
- Children should use the solution under adult supervision.
- The medical device contains small parts that may pose a choking hazard. Keep out of sight and reach of children.
Contraindications:
NASISOFT solution is contraindicated in cases of individual hypersensitivity to any of the product’s components.
Persons with individual intolerance to certain components of the solution may experience allergic reactions.
The isotonic saline solution may cause reversible bronchial constriction if inhaled by highly sensitive patients with bronchial asthma or hyperreactive bronchial systems. For particularly sensitive individuals, premedication with bronchodilators is recommended at the start of treatment to help prevent bronchospasm reactions. Premedication should be performed under medical supervision.
Discontinue use if bronchospasms or persistent coughing occur and inform your doctor.
Instructions for use:
For inhalation using a nebulizer:
- Check the expiry date on the primary packaging before use. Do not use after expiry.
- Remove one polymer dispenser containing NASISOFT solution from the strip of 10 dispensers.
- Open the dispenser by twisting the cap.
- Use the solution with a nebulizer according to the manufacturer’s instructions.
For intranasal administration:
- Check the expiry date on the primary packaging before use. Do not use after expiry.
- Remove one polymer dispenser containing NASISOFT solution from the strip of 10 dispensers.
- Open the dispenser by twisting the cap.
- Squeeze the solution from the dispenser into the nostrils.
Shelf life:
2 years in unopened packaging.
Storage conditions:
Store in a properly sealed package at a temperature between 5°C and 30°C, away from direct light and heat sources.
The product remains valid until the expiry date if stored correctly in unopened packaging.
Disposal:
Dispose of the product according to applicable household waste regulations.