Nasisoft Forte 3
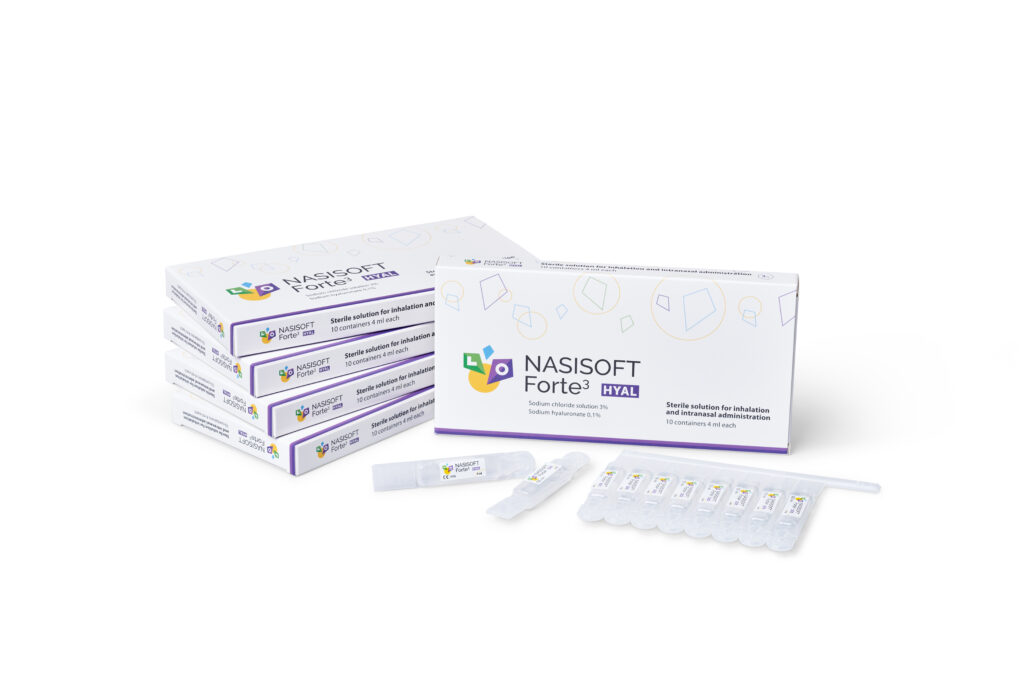
NASISOFT FORTE 3 Hyal – for thinning mucus
NASISOFT FORTE 3 Hyal is an inhalation solution with a mucolytic effect. It contains natural ingredients: a 3% sodium chloride solution and hyaluronic acid.
When used for inhalation, the 3% sodium chloride solution promotes rapid thinning and removal of mucus, while hyaluronic acid moisturizes the mucous membrane and soothes irritation, which can often cause painful coughing.
NASISOFT FORTE 3 Hyal is indicated for respiratory diseases such as bronchitis, bronchiolitis, chronic obstructive pulmonary disease (COPD), etc.
The NASISOFT FORTE 3 Hyal inhalation solution can also be used intranasally and for inhalations in cases of nasal and paranasal sinus diseases.
Thanks to its hyperosmotic action, NASISOFT FORTE 3 Hyal reduces swelling of the nasal mucosa, thereby easing nasal breathing. In addition to its moisturizing and restorative effects, hyaluronic acid prevents the adhesion (attachment) of antigens (allergens and microorganisms) to the mucosa, providing a preventive effect also in cases of allergic rhinitis.
Composition: per 1 ml of solution:
- Sodium hyaluronate – 1.0 mg
- Sodium chloride – 30.0 mg
NASISOFT FORTE 3 Hyal is a solution for inhalation and intranasal use.
Packaging: 4 ml × 10 polymer containers with sterile solution.
WHERE TO BUY?
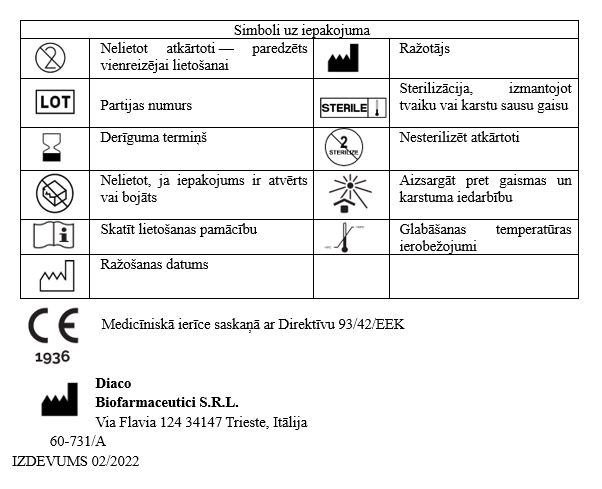
Medicīniskās ierīces NASISOFT Forte 3 Hyal lietošanas instrukcija
Nasisoft Forte 3 Hyal sterils šķīdums inhalācijām un intranazālai ievadīšanai.
Apraksts: Nasisoft Forte 3 Hyal ir sterils šķīdums inhalācijām ar inhalatoru un intranazālai ievadīšanai ar tiešu iepilināšanu deguna dobumā no polimēra dozatora. Šķīdums izstrādāti elpošanas atvieglošanai, pateicoties tā spējai sašķidrināt elpceļu sekrētu, uzlabojot tā izdalīšanos no augšējo un apakšējo elpceļu gļotādām.
Sastāvs : Nātrija hlorīds — 30,0 mg/ml, Nātrija hialuronāts — 1,0 mg/ml, Sterils ūdens
Produkta klasifikācija: Sterilizēta un apirogēna IIb klases medicīniskā ierīce.
Primārais iepakojums: 4 ml vienreiz lietojams polimēra dozators;
Sekundārais iepakojums satur: 10 polimēra dozatorus ar sterilu Nasisoft Forte 3 Hyal šķīdumu; vai 60 polimēra dozatorus ar sterilu Nasisoft Forte 3 Hyal šķīdumu.
Tikai primārā iepakojuma saturs ir sterils.
Medicīniskā ierīce sterilizēta ar tvaika sterilizāciju.
Indikācijas: Nasisoft Forte 3 Hyal ir paredzēts lietošanai visu vecumu pacientiem, kuriem diagnosticēts bronhiolīts, akūts vai hronisks bronhīts, cistiskā fibroze, hroniska obstruktīva plaušu slimība, kā arī aizdegunes un deguna dobuma patoloģijas, hronisks un sezonāls alerģisks rinīts. Šis šķīdums veicina gļotādas tūskas samazināšanu, elpceļu sekrēta sašķidrināšanu un vieglāku izvadīšanu, elpošanas atvieglošanu.
Lietošana: Inhalācijās, izmantojot inhalatoru (nebulaizeri), ievērojot inhalatora ražotāja lietošanas norādījumus. Ieteicamā deva ir viens 4 ml polimēra dozators divas reizes dienā vai saskaņā ar ārstējošā ārsta rekomendācijām. Izmantot vienu 4 ml polimēra dozatoru, atvērt to pagriežot vāciņu ar skrūvējošu kustību. Polimēra dozatora saturu iepildīt inhalatorā, ievērojot inhalatora ražotāja lietošanas pamācību ieelpot šķīduma aerosolu.
Ievadot intranazāli Izmatot vienu 4 ml polimēra dozatoru, atvērt to pagriežot vāciņu ar skrūvējošu kustību. Iespiest šķīdumu no polimēra dozatora deguna ejās. Izmantot vienu 4 ml polimēra dozatoru vienā lietošanas reizē no vienas līdz četrām reizēm dienā vai saskaņā ar ārsta rekomendācijām. Lietot šķīdumu ne ilgāk par 4 nedēļām, ja vien ārsts nav norādījis citādi.
Brīdinājumi un lietošanas piesardzības pasākumi
- Šķīdumu paredzēts lietot inhalācijās vai intranazālas ievadīšanas veidā. To nav atļauts injicēt.
- Pirmo reizi lietojot, šķīduma ievadīšana jāveic ārsta vai kvalificēta medicīniskā personāla uzraudzībā.
- Bronhu spazmu vai klepus gadījumā jāpārtrauc šķīduma lietošana un jākonsultējas ar ārstu.
- Viena polimēra dozatora saturs jāizlieto uzreiz pēc atvēršanas.
- Nelietot, ja šķīdums nav caurspīdīgs un bezkrāsains.
- Nelietot, ja polimēra dozators ir atvērts vai bojāts.
- Katrs šķīduma polimēra dozatora saturs paredzēts lietošanai vienā reizē. Neizlietotais šķīdums atvērtā polimēra konteinerā vairs nav sterils.
- Nesterilizēt atkārtoti. Atkārtota sterilizācija var izraisīt savstarpēju piesārņošanos.
- Šķīdumu nedrīkst sasaldēt.
- Šķīdums nav paredzēts iekšķīgai lietošanai.
- Bērniem šķīdums jālieto pieaugušo uzraudzībā.
- Medicīniskā ierīce satur sīkas detaļas, kas var radīt aizrīšanās risku. Uzglabājiet bērniem neredzamā un nepieejamā vietā.
Īpaši brīdinājumi :
- Nelietot pēc derīguma termiņa beigām.
- Nelietot Nasisoft Forte 3 Hyal šķīdumu kopā ar citiem šķīdumiem, jo tas var mainīt to iedarbību.
- Ja ir tendence uz aizdusu vai ir paaugstināts jutīgums, nedrīkst lietot Nasisoft Forte 3 Hyal šķīdumu bez ārsta uzraudzības.
Kontrindikācijas: Nelietot Nasisoft Forte 3 Hyal ja ir individuāla paaugstināta jutība pret jebkuru no šķīduma sastāvdaļām, to lietojot ir iespējamas alerģiskas reakcijas. Hipertoniskais sāls šķīdums var izraisīt atgriezenisku bronhu sašaurināšanos, ja to ieelpo ļoti jutīgi pacienti ar diagnosticētu bronhiālo astmu vai hiperreaktīvu bronhu sistēmu. Īpaši jutīgām personām uzsākot lietot Nasisoft Forte 3 Hyal, ieteicama premedikācija ārsta uzraudzībā ar bronhodilatatoriem, kas var palīdzēt novērst bronhu spazmas izraisošas reakcijas. Pārtrauciet lietot Nasisoft Forte 3 Hyal, ja rodas bronhu spazmas vai ilgstošs klepus, par to jāinformē ārstējošais ārsts.
Glabāšanas laiks: 2 gadi no ražošanas datuma neskartā iepakojumā.
Uzglabāt noslēgtā iepakojumā, temperatūras intervālā no +5 °C līdz +30 °C (ieskaitot), nepakļaut tiešai gaismas un siltuma avotu iedarbībai. Produkta derīguma termiņš ir spēkā, ja produkts tiek pareizi uzglabāts neskartā iepakojumā.
Iznīcināšana: saskaņā ar spēkā esošajiem normatīvajiem aktiem par sadzīves atkritumiem.